For adult patients with metastatic castration-resistant prostate cancer (mCRPC)1
Give your patients the opportunity for improved survival and slower decline of quality of life with Xofigo®1
Xofigo (+ best standard of care) is the first and only targeted alpha therapy to improve overall survival and slow down the decline of quality of life during on treatment period vs placebo + best standard of care (BSC) in mCRPC patients with symptomatic bone metastases and no known visceral metastases.1-6
Xofigo® monotherapy or in combination with luteinising hormone releasing hormone (LHRH) analogue is indicated for the treatment of adult patients with mCRPC, symptomatic bone metastases and no known visceral metastases, in progression after at least two prior lines of systemic therapy for mCRPC (other than LHRH analogues), or ineligible for any available systemic mCRPC treatment.1
To access the NCCP Chemotherapy regimen for Xofigo, please click here https://www.hse.ie/eng/services/list/5/cancer/profinfo/chemoprotocols/genitourinary/guregimens.html
NCCN, ESMO and AUA treatment guidelines also provide recommendations for Xofigo use in the mCRPC setting7-9
mCRPC patients with symptomatic bone metastases should be treated early with Xofigo® (prior to visceral metastases)7,8,10

*Xofigo is not recommended in patients with a low level of osteoblastic bone metastases. [SmPC]

These are illustrative algorithms based on the revised EU indication for Xofigo. Not all potential treatment options are shown. Concurrent use of bone health agents to treat osteoporosis or for patients with bone metastases is recommended.
ADT: androgen deprivation therapy; AR: androgen receptor; EU: European Union; LHRH: Luteinising hormone releasing hormone; mCRPC: metastatic castration-resistant prostate cancer; mHSPC: metastatic hormone-sensitive prostate cancer; nmCRPC: non-metastatic castration-resistant prostate cancer.
Use Xofigo while there is an opportunity for action before the development of visceral metastases2
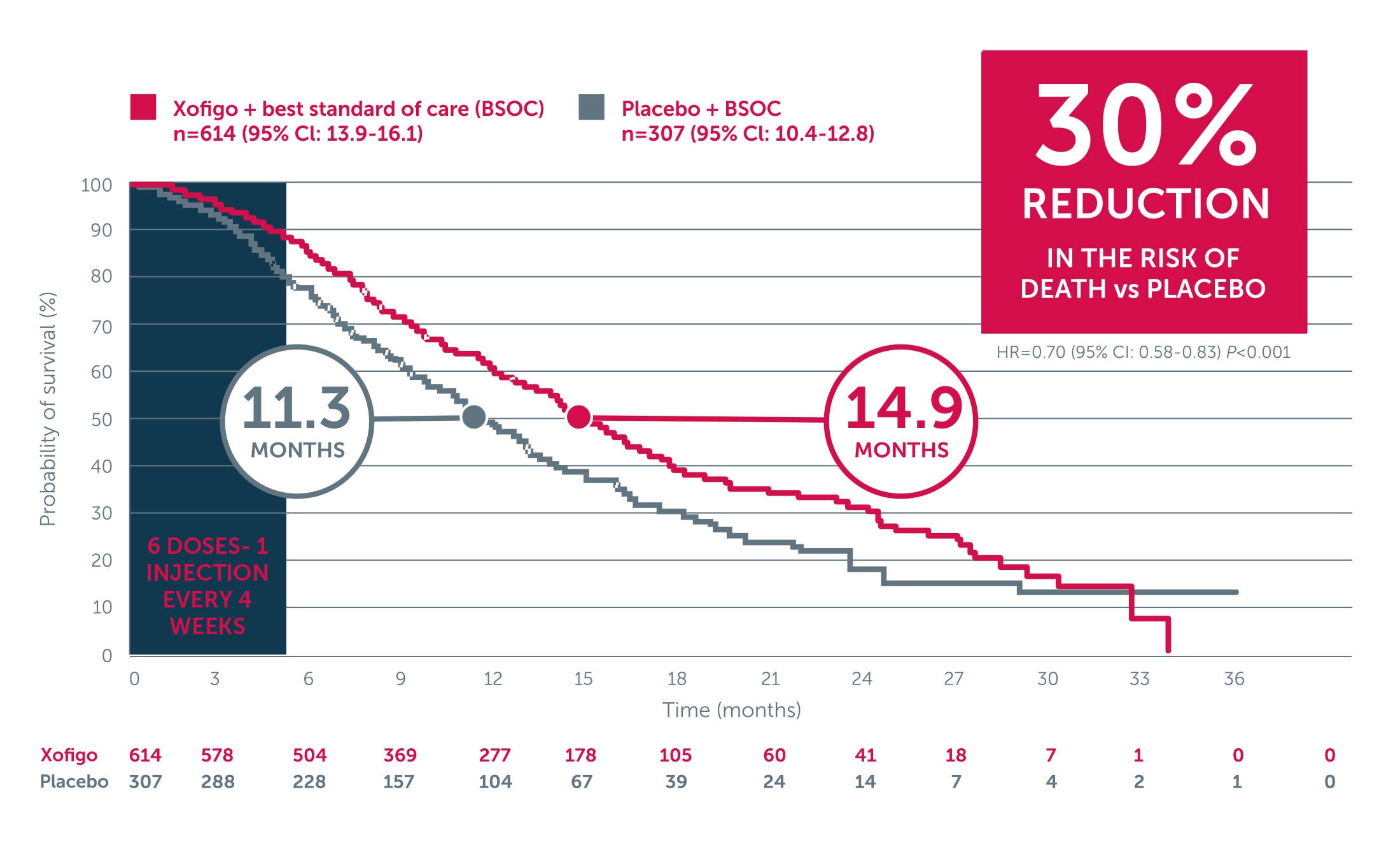
Xofigo® + BSC significantly extended median OS vs placebo + BSC1-3
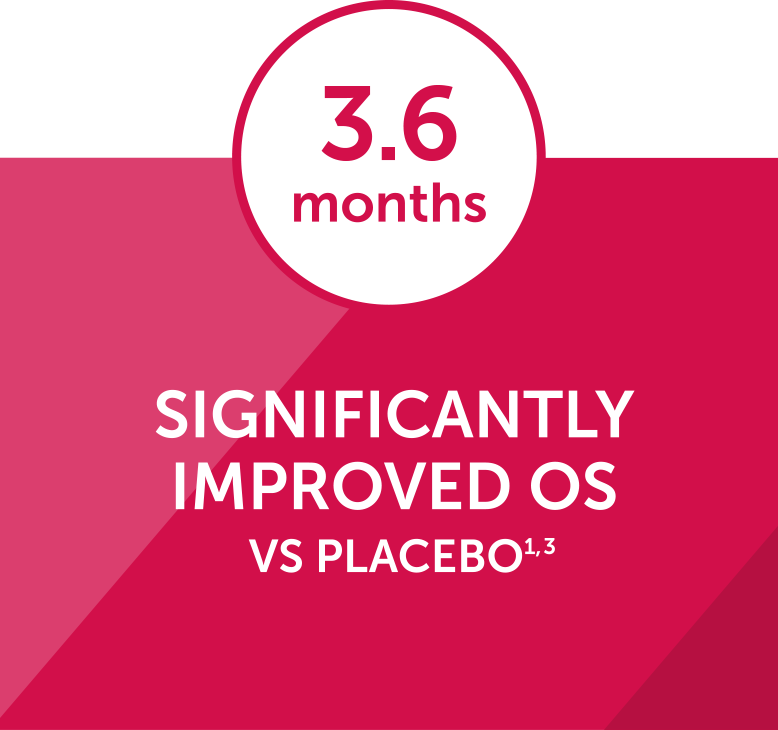
In the phase 3 ALSYMPCA trial, an updated analysis showed that Xofigo + BSC significantly extended median OS by 3.6 month vs placebo + BSC (14.9 months vs 11.3 months, respectively; P<0.001).1-3

Adapted from Parker C et al. 2013. ALSYMPCA was a double-blind, randomised, placebo-controlled, phase 3 study in 921 symptomatic mCRPC patients with 2 or more bone metastases. Patients were randomised 2:1 to receive 6 injections (one every 4 weeks) of Xofigo + BSC (n=614) or placebo + BSC (307). BSC included local EBRT or treatment with bisphosphonates, corticosteroids, antiandrogens, oestrogens, estramustine, or ketoconazole. The primary endpoint was overall survival.3
BSOC: best standard of care; EBRT: external beam radiation therapy; mCRPC: metastatic castration-resistant prostate cancer; OS: overall survival
Xofigo® + BSC significantly delays time to first symptomatic skeletal event (SSE) vs placebo + BSC1-3
In the phase 3 ALSYMPCA trial, an updated analysis of secondary endpoints showed that Xofigo + BSC significantly delayed time to first SSE by 5.8 months vs placebo + BSC (15.6 months vs 9.8 months, respectively; P<0.001).1-3

Adapted from Parker C et al. 2013. ALSYMPCA was a double-blind, randomised, placebo-controlled, phase 3 study in 921 symptomatic mCRPC patients with bone metastases. Patients were randomised 2:1 to receive 6 injections (one every 4 weeks) of Xofigo + BSC (n=614) or placebo + BSC (307). BSC included local EBRT or treatment with bisphosphonates, corticosteroids, antiandrogens, oestrogens, estramustine, or ketoconazole. SSEs were defined as EBRT for pain relief, spinal cord compression, tumour-related orthopaedic surgical interventions, bone fractures. The primary endpoint was overall survival.3
BSOC: best standard of care; EBRT: external beam radiation therapy; mCRPC: metastatic castration-resistant prostate cancer; OS: overall survival; SSE: symptomatic skeletal event.
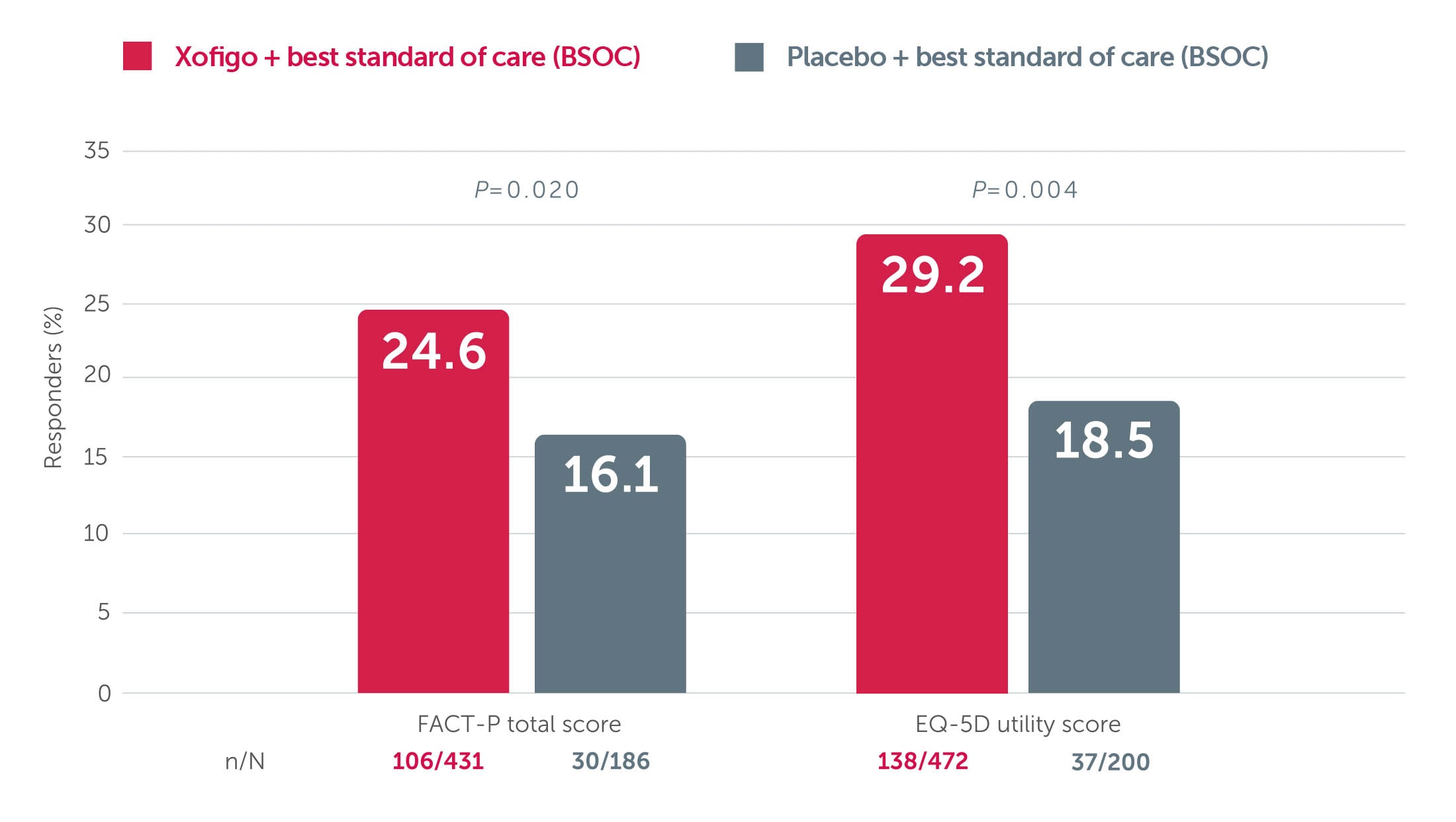
Improved survival with Xofigo® + BSC is accompanied by a slower decline in quality of life vs placebo + BSC1,3,4
A post hoc analysis of the phase 3 ALSYMPCA trial showed that significantly more patients treated with Xofigo + BSC experienced a meaningful improvement in quality of life (as measured by FACT-P total score or EQ-5D utility score) vs placebo + BSC (P=0.02 and P=0.004, respectively).1,3,4
In addition, significantly more patients experienced pain relief with Xofigo + BSC than placebo + BSC (30.2% [129/427] vs 20.1% [37/184], respectively; P=0.01)16

Adapted from Nilsson S et al. 2016. ALSYMPCA was a double-blind, randomised, placebo-controlled, phase 3 study in 921 symptomatic mCRPC patients with bone metastases. Patients were randomised 2:1 to receive 6 injections (one every 4 weeks) of Xofigo + BSC (n=614) or placebo + BSC (307). BSC included local EBRT or treatment with bisphosphonates, corticosteroids, antiandrogens, oestrogens, estramustine, or ketoconazole. The primary endpoint was overall survival.3 A post hoc analysis looked at health-related quality of life using two validated instruments: the general EQ-5D and the disease-specific FACT-P.4 Meaningful improvement defined as increase in FACT-P total score of ≥10 from baseline or an increase in EQ-5D utility score of ≥0.1 from baseline, at Week 16 and/or Week 24.
BSOC: best standard of care; EBRT: external beam radiation therapy; EQ-5D: EuroQoL 5D; FACT-P: Functional Assessment of Cancer Therapy-Prostate; mCRPC: metastatic castration-resistant prostate cancer.
Safety profile of Xofigo® + BSC was comparable to placebo + BSC3
In the phase 3 ALSYMPCA trial, the rates of AEs were similar with Xofigo + BSC vs placebo + BSC.3

ALSYMPCA was a double-blind, randomised, placebo-controlled, phase 3 study in 921 symptomatic mCRPC patients with bone metastases. The safety population included 600 patients in the Xofigo + BSC group and 301 patients in the placebo + BSC group.3
- The most frequently observed adverse reactions (≥ 10%) in patients receiving Xofigo were diarrhoea, nausea, vomiting, thrombocytopenia and bone fracture1
- The most serious adverse reactions were thrombocytopenia and neutropenia1
- Xofigo increases the risk of bone fractures. In clinical studies, concurrent use of bisphosphonates or denosumab reduced the incidence of fractures in patients treated with Xofigo1
For the full list of AEs, please refer to the medicines.ie
Treatment considerations
- Chemotherapy may be used after Xofigo1,10,13
*The haematological safety profiles for patients receiving chemotherapy after Xofigo were similar to those seen in patients receiving chemotherapy after placebo.13
Patient considerations
- Low incidence of Grade 3–4 vomiting3,14
- Can be administered without the need for steroids†
†Xofigo is contraindicated in combination with abiraterone acetate and prednisone/prednisolone.1 Concomitant use of steroids may further increase the risk of fracture.1
AE: adverse event; BSOC: best standard of care; mCRPC: metastatic castration-resistant prostate cancer.
Xofigo® has a fixed course of treatment1
Xofigo is administered as a 1-minute IV injection every 4 weeks for 6 injections.1

Treat for all 6 cycles of Xofigo to maximise overall survival benefit1,15
Standard bloodwork required prior to every dose.
Help your patients experience the benefits of Xofigo1,3,4


Use of Xofigo is not recommended in patients with low levels of osteoblastic bone metastases.1
REGISTER FOR E-COMMUNICATION
REGISTER FOR EVENTS
PP-XOF-IE-0061-3, August 2021
Reporting adverse events and quality complaints
Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigiliance. Reports can also be sent directly to Bayer via this link. Both side effects or quality complaints can be reported to Bayer by email to adr-ireland@bayerhealthcare.com