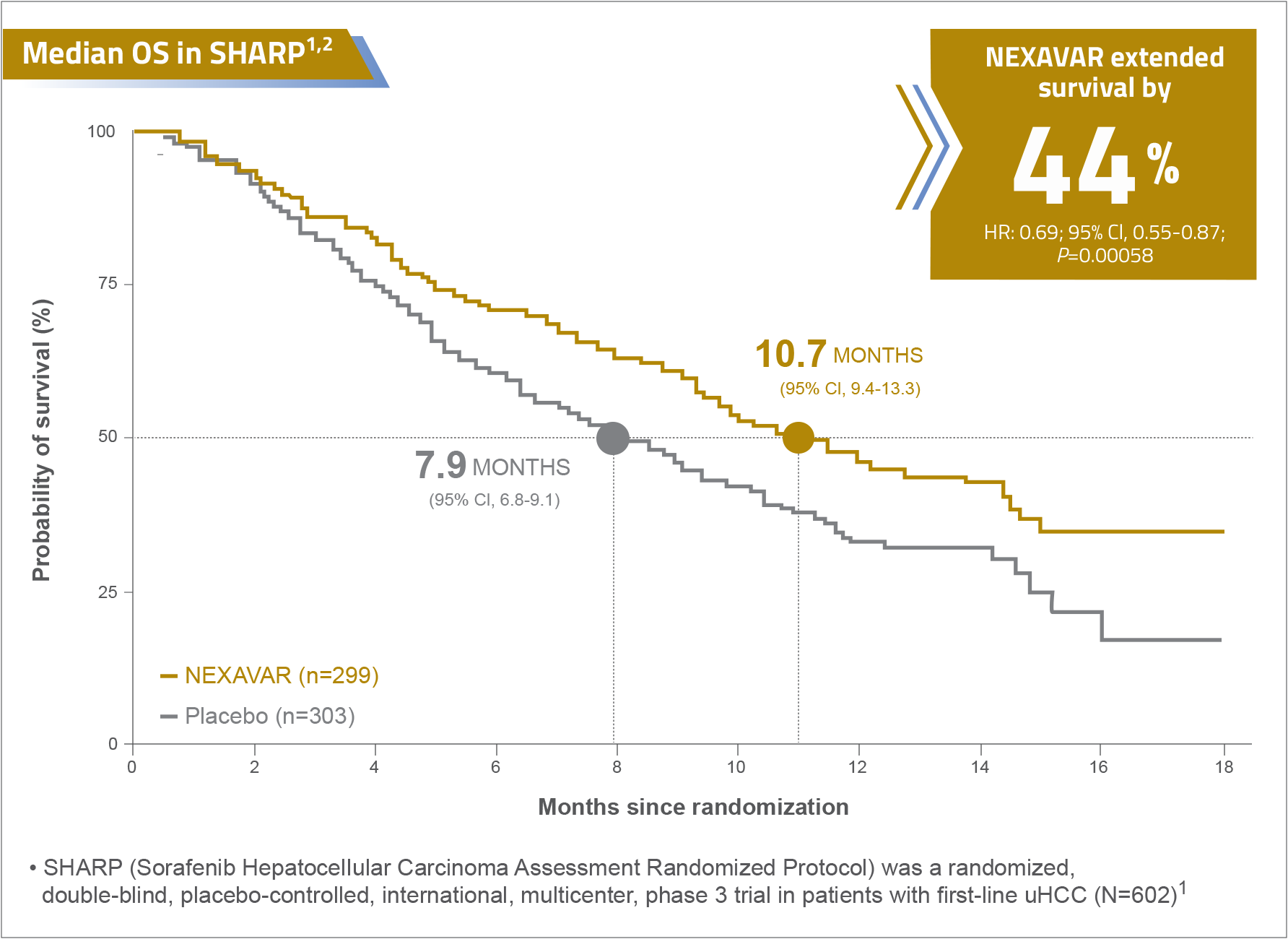
Proven Efficacy in Phase III randomised controlled trial
NEXAVAR® (sorafenib) provides a proven OS benefit in first-line uHCC treatment1,2
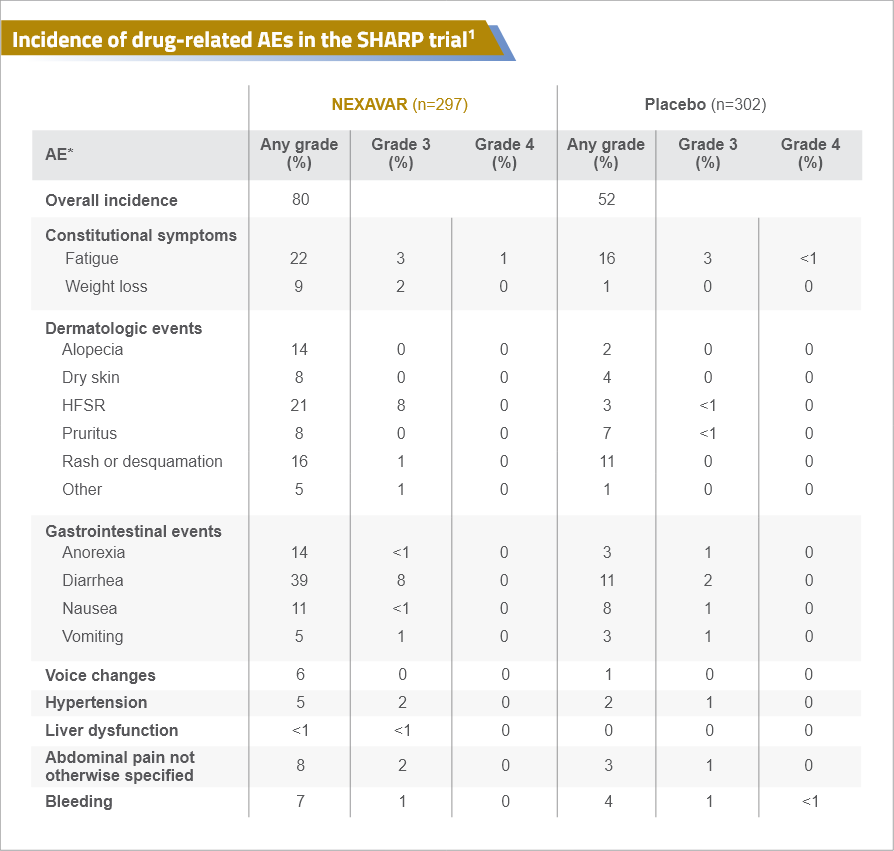
Safety Profile of Nexavar from the Phase III SHARP trial
NEXAVAR® (sorafenib) has a predictable and manageable safety profile1
REGISTER FOR E-COMMUNICATION
REGISTER FOR EVENTS
PP-NEX-IE-0017-3, July 2023
Reporting adverse events and quality complaints
Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigiliance. Reports can also be sent directly to Bayer via this link. Both side effects or quality complaints can be reported to Bayer by email to adr-ireland@bayerhealthcare.com


