For adult patients with non-metastatic castration-resistant prostate cancer (nmCRPC) who are at high risk of developing metastatic disease1
Introducing NUBEQA® – significantly delays metastasis with Androgen Deprivation Therapy vs. Androgen Deprivation Therapy alone, without increasing the rate of treatment discontinuation due to adverse events1,2
For men with nmCRPC who are at high risk of developing metastatic disease, progression to mCRPC is a tipping point for increased sufering and mortality.3-9 NUBEQA is a novel androgen receptor inhibitor for nmCRPC that extends median metastasis-free survival (MFS) without increasing the rate of treatment discontinuation due to adverse events vs. ADT alone.1-3
The benefits of NUBEQA have been demonstrated in the ARAMIS study, the largest phase 3 study of nmCRPC to date (N=1,509).1,2,10,11
NUBEQA® is indicated for the treatment of adult men with non-metastatic castration-resistant prostate cancer (nmCRPC) who are at high risk of developing metastatic disease1
Do current therapies help you achieve the treatment goals of men with nmCRPC?
Delay metastasis12,15
NUBEQA + ADT delivers:
40.4 months MFS - more than double the median MFS of ADT1,2
Maintain your patient’s current lifestyle13
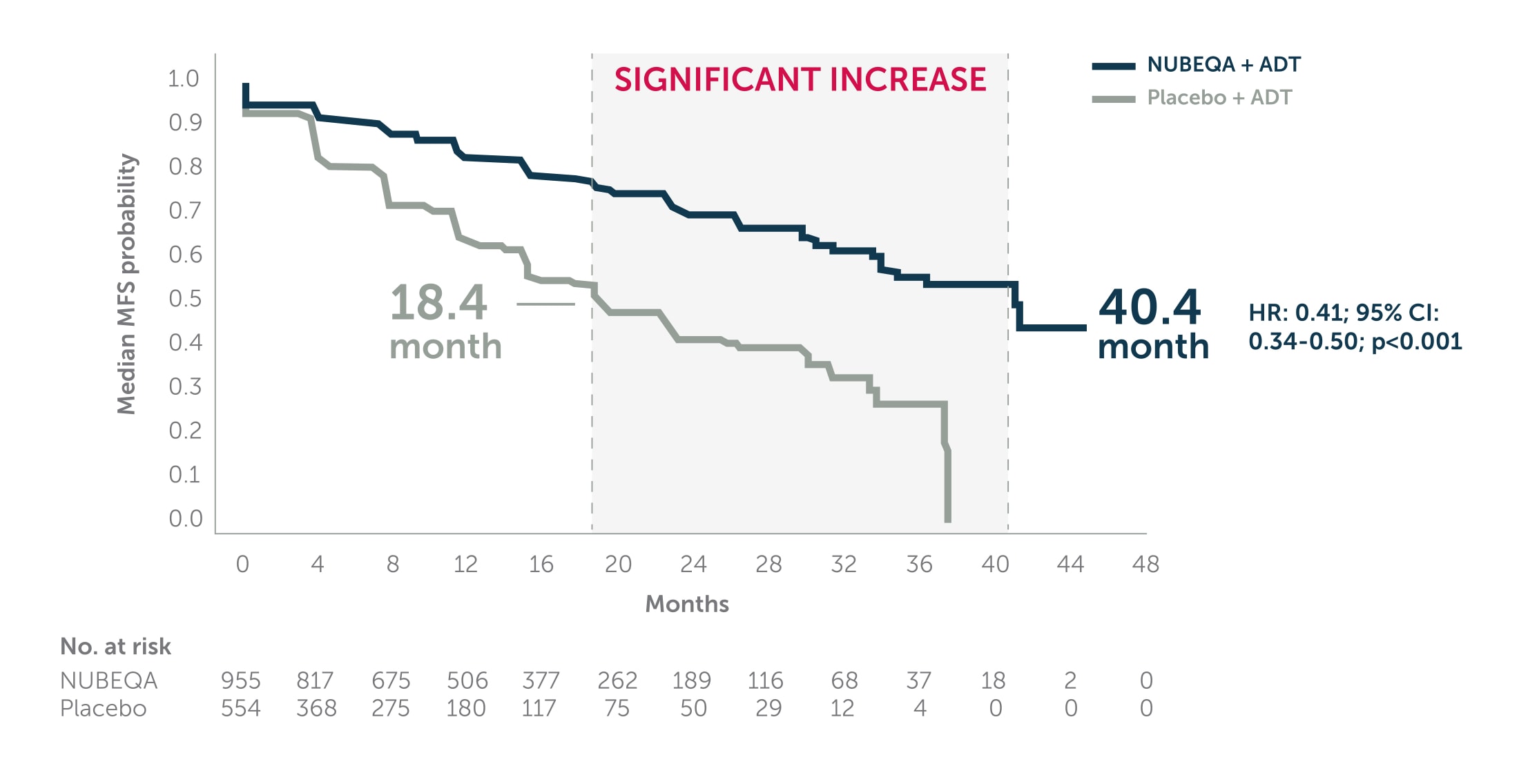
NUBEQA® + ADT more than doubled median MFS vs ADT1,2
In the phase 3 ARAMIS trial, NUBEQA + ADT significantly extended median MFS by 22 months vs ADT (40.4 months vs. 18.4 months, HR: 0.41; 95% CI:0.34–0.50; p<0.001).1,2
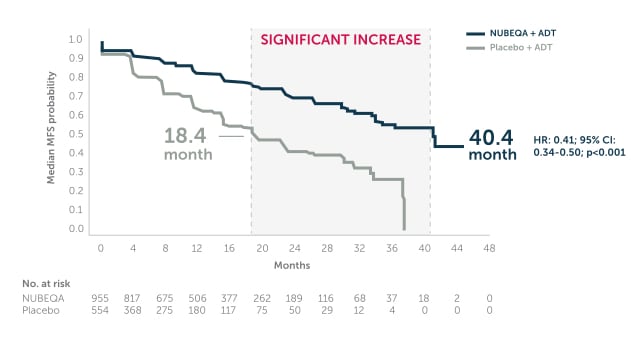
Adapted from Fizazi K et al. 2019. ARAMIS is a double-blind, randomised, placebo-controlled, multicentre phase 3 study in 1,509 nmCRPC patients with PSA doubling time of ≤ 10 months. Patients were randomised 2:1 to receive NUBEQA + ADT 600 mg bd (n=955) or placebo + ADT (n=554). The primary endpoint was metastasis-free survival. The planned primary analysis was performed after 437 primary end-point events had occurred.2
ADT: androgen deprivation therapy; bd: twice daily; MFS: Metastasis Free Survival; nmCRPC: non-metastatic castration-resistant prostate cancer; PSA: prostate-specific antigen.
NUBEQA® + ADT significantly improved overall survival vs. ADT alone14
In the phase 3 ARAMIS trial, the pre-specified final overall survival analysis showed that NUBEQA + ADT significantly reduced the risk of death by 31% vs ADT alone (HR=0.69, 95% CI 0.53–0.88; p=0.003).14

Adapted from Fizazi et al. 2020. ARAMIS is a double-blind, randomised, placebo-controlled, multicentre phase 3 study in 1,509 nmCRPC patients with PSA doubling time of <10 months. Patients were randomised 2:1 to receive NUBEQA + ADT 600 mg bd (n=955) or placebo + ADT (n=554). The primary endpoint was metastasis-free survival.2 These data are from the pre-specified final OS analysis as OS data were not yet mature at the time of the MFS analysis.14
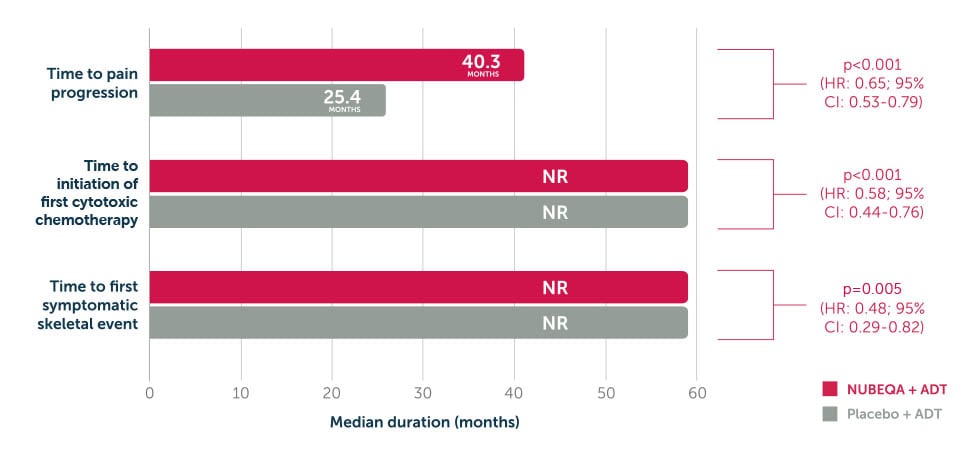
In the phase 3 ARAMIS trial, the pre-specified final analysis showed that adding NUBEQA® to ADT significantly prolonged the time to each key secondary endpoint14
- Time to pain progression
- Time to initiation of first chemotherapy
- Time to first symptomatic skeletal event.

Adapted from Fizazi et al. 2020. ARAMIS is a double-blind, randomised, placebo-controlled, multicentre phase 3 study in 1,509 nmCRPC patients with PSA doubling time of <10 months. Patients were randomised 2:1 to receive NUBEQA + ADT 600 mg bd (n=955) or placebo + ADT (n=554). The primary endpoint was metastasis-free survival.2 Time to pain progression was evaluated using data from the primary analysis cut -off date of September3, 2018; data for time to initiation of chemotherapy and time to first symptomatic skeletal event are from the pre-specified final analysis (data cut-off November 15, 2019).14
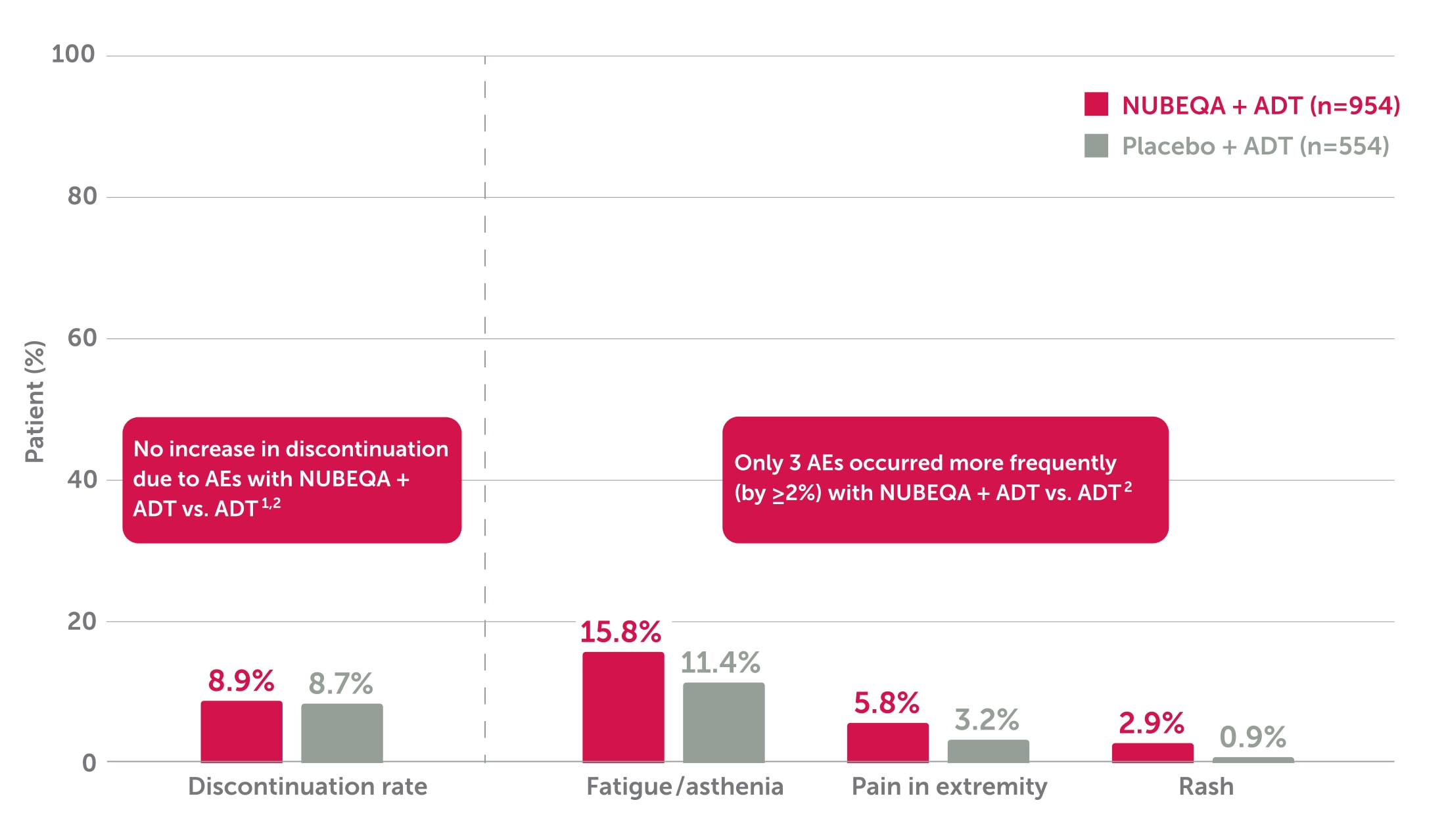
Adding NUBEQA® to ADT did not increase the discontinuation rate due to AEs vs ADT2
In the phase 3 ARAMIS trial, there was no increase in discontinuation due to AEs with NUBEQA + ADT vs ADT (8.9% vs. 8.7%).2

Adapted from Fizazi K et al. 2019. ARAMIS is a double-blind, randomised, placebo-controlled, multicentre phase 3 study in 1,509 nmCRPC patients with PSA doubling time of <10 months. The safety population included 954 patients in the NUBEQA + ADT group and 554 patients in the placebo + ADT group.2
- The most frequently observed adverse reactions in patients receiving NUBEQA were fatigue/asthenic conditions (≥1/10) and rash, pain in the extremity, musculoskeletal pain, fractures, ischaemic heart disease and heart failure (≥1/100)1,2
- Three laboratory test abnormalities were reported more frequently with NUBEQA + ADT than ADT:1
- Neutrophil count decreased (19.6% vs. 9.4%)
- Bilirubin increased (16.4% vs. 6.9%)
- AST increased (22.5% vs. 13.6%)
- With the exception of hypertension and urinary retention, there was very low incidence (<1%) of grade 3 or 4 AEs with NUBEQA + ADT2
- NUBEQA + ADT vs. ADT alone: Hypertension (3.1% vs. 2.2%); urinary retention (1.6% vs. 2.0%)
- With extended follow-up, the safety profile NUBEQA was favourable and consistent with the primary anaylsis14
For further information, please refer to the Summary of Product Characteristics.
ADT: androgen deprivation therapy; AE: adverse event; AST: aspartate aminotransferase.
Dosing for NUBEQA®1
Two 300 mg tablets, twice daily with food and water.1

Total daily dose 1200 mg.1
- No dose adjustment is necessary for patients with mild or moderate renal impairment or mild hepatic impairment1
For more information please refer to the Summary of Product Characteristics.
Add NUBEQA® to ADT to significantly delay metastasis and improve overall survival vs. ADT alone, without increasing the rate of treatment discontinuation due to adverse events1,2

REGISTER FOR E-COMMUNICATION
REGISTER FOR EVENTS
PP-PF-ONC-IE-0029-4, March 2022
Reporting adverse events and quality complaints
Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigiliance. Reports can also be sent directly to Bayer via this link. Both side effects or quality complaints can be reported to Bayer by email to adr-ireland@bayerhealthcare.com