Extended OS proven in phase 3, randomized, controlled trials

CORRECT, COloRectal cancer treated with REgorafenib or plaCebo after failure of standard Therapy; OS, overall survival. CONCUR; Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer.
Tolerability profile of Stivarga from Phase 3 CORRECT Trial
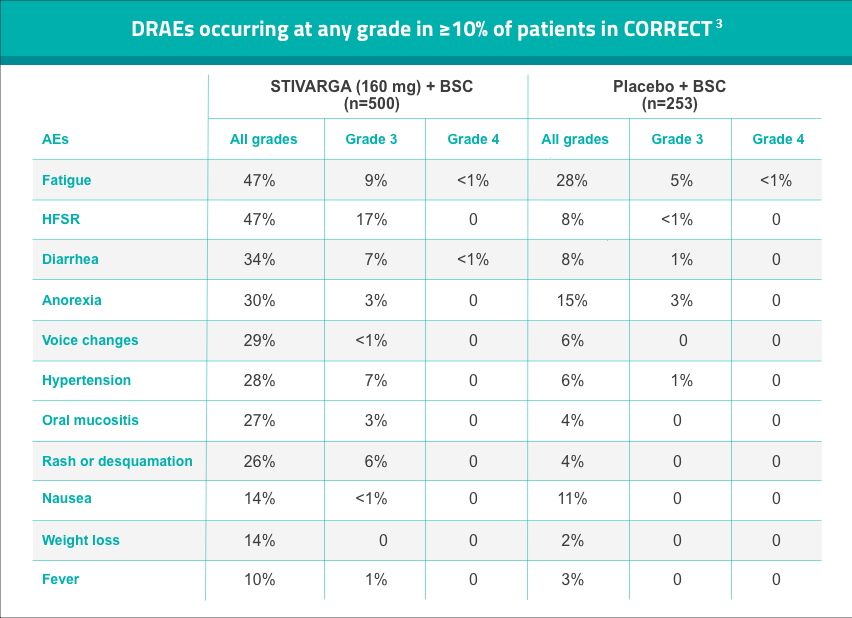
AE, adverse event; ALT, alanine transaminase; AST, aspartate transaminase; CORRECT, COloRectal cancer treated with REgorafenib or plaCebo after failure of standard Therapy; DRAE, drug-related adverse event; HFSR, hand-foot skin reaction.
Mechanism of Disease
REGISTER FOR E-COMMUNICATION
REGISTER FOR EVENTS
PP-STI-IE-0016-4, August 2024
Reporting adverse events and quality complaints
Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigiliance. Reports can also be sent directly to Bayer via this link. Both side effects or quality complaints can be reported to Bayer by email to adr-ireland@bayerhealthcare.com


